FAQ
Optiscan Technology
How it works
What is Optiscan technology?
Our patented Optiscan technology is carefully constructed under a microscope. Our precision engineers have miniaturised the conventional benchtop confocal microscope into a handheld clinical grade imaging probe providing single-cell imaging capability in the palm of your hand.
We have revolutionised the fibre optic confocal laser endomicroscopy platform by introducing point scanning high resolution handheld microscopes with dynamic optical sectioning capability to our imaging probes. Our technology provides histopathology equivalent digital images for live clinical and life-sciences imaging and enables cellular and single-cell level virtual biopsy directly from living tissue. All of this is slide-free, biopsy-free, and non-destructive, unlike traditional histopathology.
What is Confocal Laser Endomicroscopy (CLE)?
Endomicroscopy (endo: introduced into the body, microscopy: visualisation of microscopic objects) is a technique for obtaining histology-like images from living tissue. Endomicroscopy is also known as optical biopsy, virtual histology, virtual biopsy, fibre optic confocal microscopy, etc.
How is Optiscan technology different from other CLE devices?
Optiscan Technology:
Optiscan’s devices use a scanned optical fibre to physically scan the tip of the optical fibre in a raster pattern across the back of the objective lens in the scanner, projecting a diffraction limited spot into the sample being imaged. Fluorescent light excited at the focussed spot is captured by the objective lens and focussed back into the optical fibre and transmitted to a detector. The lateral optical resolution (0.55µm) is matched to the digital sampling rate to collect megapixel images that adequately sample the area being imaged (475x475µm). The scanner z-focus enables movement of the focal plane that are smaller (3µm) than the axial resolution (5.1µm), enabling adequate sampling of thin optical sections in the z-dimension.
Other CLE technologies:
There are other CLE devices in use. Fibre bundle, MEMS scanning devices falls under the banner of CLE.
Fibre Bundle:
This mechanism produces images by sequentially scanning individual optical fibre cores in the proximal (processor) end a coherent fibre optic bundle and projecting light into the sample through a lens on the distal (tissue) end of the bundle. While fibre bundles allow for very small diameter probes with no moving parts at the distal end, they sacrifice resolution and do not allow variable imaging depth – an essential feature of high-resolution confocal microscopy. The digital image resolution of fibre bundles is limited due to the small number of fibres in the bundle resulting in small fields of view, under sampling, and reduced sensitivity due to the spacing between the cores of the fibre. Individual fibre cores in bundles break, limiting the life of the probe.
MEMS scanning:
Devices which use scanning mirrors have been trialled, but are large in size, or fragile and have small fields of view.
In what way is Optiscan different from a regular confocal microscope?
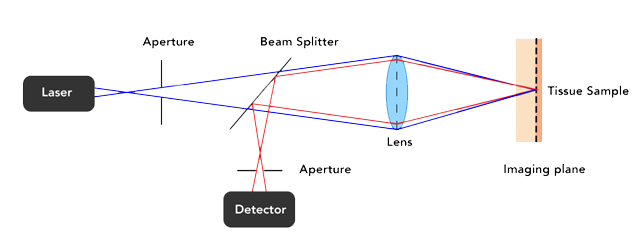
In conventional confocal microscopes. the scanning and optical filtering of light typically requires bulky optics and long beam paths meaning conventional confocal microscopes are large, bench mounted devices that require a stable platform to retain their precise optical alignment. Samples are typically put onto the stage of a regular optical microscope to be imaged.
Confocal microscopes scan a point of light across a sample and measure the intensity of light that returns from that spot. They use a pinhole to block light returning from parts of the sample outside the spot and can therefore generate high contrast images from thick and living samples.
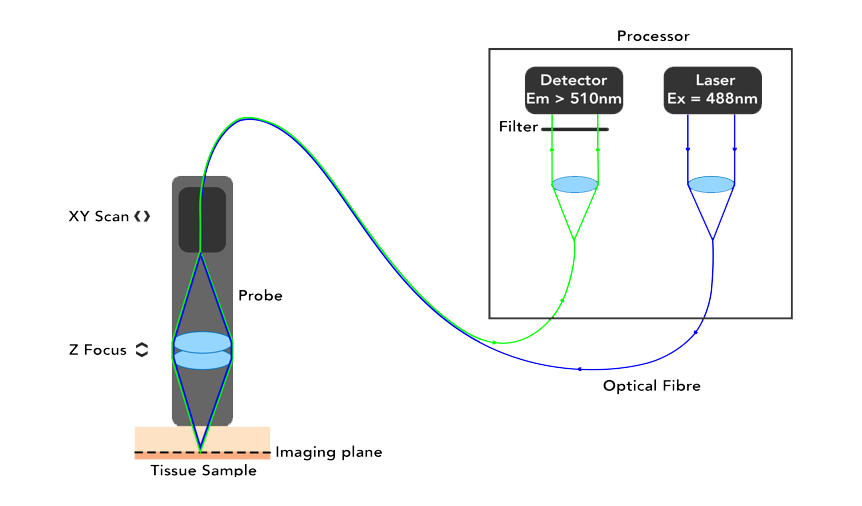 Optiscan uses a single optical fibre tip as the confocal pinhole. A miniaturised optical fibre scanning mechanism scans the fibre tip to generate an image. The endomicroscopes are small, handheld devices that can be touched against a sample of interest. The contact between the probe and the sample provides stability and a z-plane reference so that megapixel images with submicron resolution can be easily captured from large and living samples.
Optiscan uses a single optical fibre tip as the confocal pinhole. A miniaturised optical fibre scanning mechanism scans the fibre tip to generate an image. The endomicroscopes are small, handheld devices that can be touched against a sample of interest. The contact between the probe and the sample provides stability and a z-plane reference so that megapixel images with submicron resolution can be easily captured from large and living samples.
Imaging
Can I image in real time?
Yes. Images are displayed on the screen and also collected instantaneously in the background. All images can be saved for later viewing and analysis.
Can I see single-cell structures?
Yes. The Optiscan technology offers the highest resolution of all endomicroscope devices. It has a lateral resolution of 0.55µm, and an axial resolution (optical slice thickness) of 5.1µm. Not only is it possible to clearly image single cell structures, but it is also possible (with appropriate labelling agents) to image sub-organelle structures in vivo.
How fast can I capture images?
Images can be captured images from 0.8 to 3.5 frames per second, depending on the aspect ratio and the number of lines collected per image.
Does the endomicroscope probe need to touch the tissue of interest?
The endomicroscope is designed to press gently on the sample to be imaged, and then move the focal plane in the probe to locate the region of interest. This gentle pressure provides imaging stability and “wet contact” with the sample provides a good optical interface. It is possible to image without touching the probe to the sample, but imaging in this configuration will be prone to movement artefact, as microscopic movement in the x, y or z axis between the probe and the sample will be reflected in the image.
Does contact pressure affect imaging depth?
The endomicroscope only needs to touch the tissue lightly when imaging. It is not necessary to apply much pressure. Varying pressure does not affect imaging depth and (unless excessive) does not distort the resulting generated images. The imaging depth is varied by the operator via a foot pedal or mouse control that moves the optics within the distal tip of the ViewnVivo probe.
Can I scan through the Z Plane with Optiscan’s devices?
Yes, our technology allows the user to dynamically control the Z-focus depth over a range of up to 400µm. The imaging depth can be changed in single step sizes as defined by the user, or by 10µm per second during continuous adjustment. Z-stacks can be captured, with operator-settable depth.
Laser and Filter
What wavelength laser is used in the Optiscan technology?
It is a single channel confocal microscope with 488nm blue laser illumination. It detects the fluorescent emission across a broad bandwidth onto a single detector. Multiple fluorophores can be unmixed by sequential imaging of the same region through optical filters that transmit light from discrete parts of the spectrum.
It is also possible to use multiple fluorophores that label different structures within a tissue and identify fluorophore binding based on morphology. The ViewnVivo is equipped with 8 preinstalled and 4 customizable filters. These filters facilitate spectral differentiation of fluorophores.
What fluorophore are compatible with the Optiscan technology?
The Optiscan scanners can image any blue (488nm) excited fluorophore that emits in the visible and near infrared part of the spectrum (515nm – 750nm). 488nm illumination is in an optical ‘sweet spot’, exciting many fluorophores and offering higher resolution imaging than lasers with longer wavelengths, while still enabling reasonable depth penetration for sub surface imaging.
Is the laser safe?
Optiscan uses a low power divergent laser. Output from the probe is between 10-1000µW. The laser is safe for live clinical and life science imaging. General laser safety precautions apply such as wearing laser safety goggles, avoiding looking into the probe tip with optical magnifiers while it is scanning. Refer to product manual for more information.
We encourage users to refer to the product manual for more information.

